Adrenal Tumor:
At 36, Ana* thought she finally understood her body.
For months, she had been living in silent discomfort: painful acne that no makeup could hide, thick dark hairs sprouting along her chin and cheeks, an unsettling disappearance of her menstrual periods, and a blood pressure that wouldn’t stop climbing.
Her gynecologist was quick to explain it away:
“It’s PCOS. Nothing unusual. Let’s just put you on birth control pills and everything will regulate itself.”
Ana wanted to believe it.
She clutched that prescription like a lifeline, faithfully taking the pills day after day, hoping her body would calm down, her skin would heal, her life would steady itself.
But six months passed — and instead of healing, her body was screaming louder.
Her acne worsened.
The hair on her face grew thicker.
Her periods, though artificially controlled by the pills, felt unnatural.
And the heaviness in her lower back—a dull, nagging pain she chalked up to stress—became impossible to ignore.
In her heart, Ana knew:
This wasn’t normal.
Adrenal Tumor: When the Body Speaks — and No One Listens
Frustrated and scared, Ana sought a second opinion.
This time, she found a reproductive endocrinologist who, instead of handing her a prescription in five minutes, sat back, listened, and started asking real questions.
He didn’t just see her symptoms.
He saw her.
“Your story doesn’t fit the usual pattern of PCOS,” he said carefully. “Let’s dig deeper.”
He ordered a full panel of hormone tests:
- Total testosterone
- DHEA-S (dehydroepiandrosterone sulfate)
- Cortisol
- Prolactin
- TSH (thyroid-stimulating hormone)
Ana left the clinic clinging to a fragile hope: that finally, someone was taking her seriously.
When the results came back, they changed everything.
Adrenal Tumor: The Hidden Monster
Ana’s DHEA-S level was over 900 mcg/dL — more than three times the normal upper limit.
It wasn’t just slightly elevated.
It was screaming something was wrong.
“DHEA-S is mainly produced by the adrenal glands,” the doctor explained.
“When it’s this high, we need to investigate more closely. It could mean something serious.”
Then came the question that broke through Ana’s confusion:
“Have you been feeling any kind of back pain? Lower back, one side more than the other?”
Ana froze.
Yes.
That pain had been her silent companion for months, pushed aside as “normal” stress.
It wasn’t normal. It was a warning sign.
Adrenal Tumor: Unveiling the Truth
The doctor ordered a CT scan immediately.
The images revealed the truth:
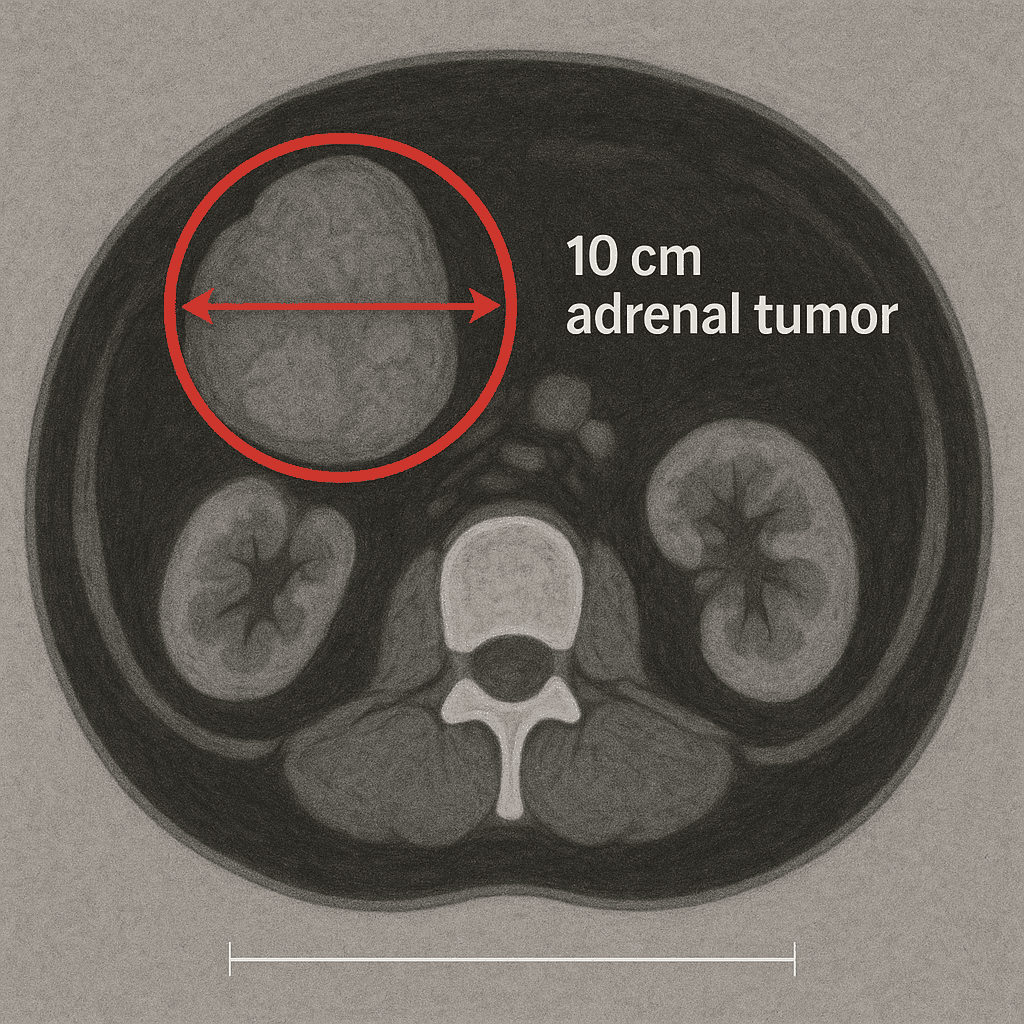
A giant tumor, 14 x 10 x 10 centimeters, sitting in Ana’s left adrenal gland.
Solid, well-defined — everything suggesting a benign lesion, but so large it needed urgent removal.
The tumor wasn’t just sitting there quietly.
It was actively producing androgens and cortisol, flooding Ana’s body with hormones that twisted her skin, her cycles, her blood pressure, her very sense of self.
Ana had spent six months fighting a phantom diagnosis.
All the while, this monster inside her grew, hidden just beyond reach.
Adrenal Tumor: The Race to Save Her Health
The decision was made quickly:
Ana would undergo a laparoscopic adrenalectomy, using a hand-assisted technique to manage the massive size of the tumor safely.
The surgery was delicate.
- Surgeons approached the adrenal gland through a minimally invasive method.
- A Pfannenstiel incision allowed the tumor to be carefully extracted without rupturing it.
- Blood loss was minimal.
- No blood transfusion was needed.
- She was closely monitored with corticosteroids (prednisone) post-operatively to balance her hormones safely.
Five days later, Ana walked out of the hospital — lighter, freer, healing.
Adrenal Tumor: Healing What Was Lost
The transformation was slow, but beautiful.
Month after month, Ana’s body reclaimed itself:
- Her menstrual cycles returned naturally.
- Her skin began to clear.
- The facial hair growth slowed, then softened, then nearly disappeared.
- Her blood pressure stabilized without medication.
- The constant ache in her back was gone — like a ghost exorcised.
For the first time in years, she could look in the mirror without feeling like a stranger was staring back.
Adrenal Tumor: Lessons from Her Journey
Ana’s story isn’t just about a tumor.
It’s about trusting your body when something feels wrong, even if others dismiss it.
It’s about asking more questions, even if the first answer seems easy.
It’s about not settling for “common” explanations when your instincts scream “this isn’t me.”
Adrenal Tumor: Medical Discussion
Many women receive a diagnosis of PCOS (Polycystic Ovary Syndrome) based on symptoms like acne, irregular periods, and excess hair growth. PCOS is common, affecting up to 10% of reproductive-age women, but it’s not the only explanation for these symptoms. Sometimes, behind what looks like a classic PCOS case, there’s something far more serious hiding — an adrenal tumor.
An adrenal tumor is a growth on one of the adrenal glands, which sit above each kidney and produce essential hormones like cortisol, adrenaline, and androgens. Some adrenal tumors are nonfunctional, meaning they don’t secrete hormones. But others, called functioning adrenal tumors, release excessive amounts of hormones — especially androgens like DHEA-S — that mimic the hormonal imbalance seen in PCOS. These tumors can be benign or malignant, but even benign ones can wreak havoc on a woman’s health if not identified early.
How Adrenal Tumors Mimic PCOS
PCOS typically develops slowly over years. A girl might first notice irregular menstrual cycles during her teens. Acne and slight hair growth may gradually follow. The ovaries, when examined by ultrasound, usually show multiple small follicles that confirm the diagnosis. Blood tests may reveal slightly elevated testosterone levels and other mild hormonal imbalances. These findings, taken together, form the typical picture of PCOS.
However, when symptoms emerge rapidly, with unusual intensity, doctors should consider alternative causes — especially an adrenal tumor.
Let’s take Ana’s case. Ana was 27 when she started developing sudden facial hair, deep acne, and irregular menstruation. Within just three months, she felt as if her body was changing overnight. Her doctors initially assumed PCOS and started standard treatment. But something didn’t add up. Her ultrasound showed normal ovaries. Her testosterone levels were slightly elevated, but one hormone stood out — DHEA-S was extremely high.
This hormone, DHEA-S, is mainly produced by the adrenal glands, not the ovaries. Extremely elevated levels are a hallmark of an adrenal tumor.
In Ana’s case, further testing confirmed the presence of a 9.8 cm adrenal tumor, which was the true cause of her symptoms. The PCOS diagnosis had delayed her proper treatment.
Key Differences Between PCOS and Adrenal Tumors
- Onset: PCOS develops gradually. An adrenal tumor causes a sudden spike in symptoms.
- Ovarian appearance: Polycystic ovaries are typical in PCOS. With an adrenal tumor, the ovaries are usually normal.
- Hormones: Mild elevation of testosterone is common in PCOS. Extremely high DHEA-S strongly suggests an adrenal tumor.
- Imaging: A pelvic ultrasound may be unremarkable in adrenal cases. An abdominal CT or MRI is needed to detect an adrenal tumor.
- Progression: PCOS is chronic and usually stable. An adrenal tumor may grow rapidly and cause worsening symptoms.
Red Flags That Should Prompt Further Evaluation
Physicians need to be alert to warning signs that suggest a condition beyond PCOS. These include:
- Rapid onset of acne or hirsutism
- Amenorrhea or menstrual irregularities with sudden onset
- Lower back pain or flank discomfort
- Central obesity, high blood pressure, or signs of cortisol excess
- A family history of endocrine tumors or genetic syndromes
- Laboratory values showing extremely high DHEA-S
In all these situations, the possibility of an adrenal tumor must be evaluated seriously.
The Right Work-Up
To rule out or confirm an adrenal tumor, a physician should order a complete hormonal panel, which typically includes:
- Total and free testosterone
- DHEA-S
- 17-hydroxyprogesterone
- Cortisol (including 24-hour urine cortisol or dexamethasone suppression test)
- TSH and prolactin (to exclude other endocrine causes)
If DHEA-S is elevated, imaging tests such as an abdominal CT scan or MRI are the next steps. These images can identify whether an adrenal tumor is present, and also determine its size, shape, and likelihood of malignancy.
How Adrenal Tumors Are Treated
Most benign adrenal tumors are treated surgically. The standard approach today is laparoscopic adrenalectomy — a minimally invasive surgery to remove the tumor and affected adrenal gland. This technique has a high success rate, minimal recovery time, and low complication risk.
In cases where the adrenal tumor is large (over 6 cm) or has suspicious features, a more extensive surgery may be required. Some hospitals use hand-assisted laparoscopic techniques to remove large tumors without fully opening the abdomen.
After tumor removal, most hormone levels normalize within weeks. The resolution of symptoms can be striking — acne clears, hair growth slows, and periods often return to regularity.
But follow-up care is essential. If both adrenal glands are affected, patients may need lifelong hormone replacement. Even when only one gland is removed, some women experience temporary adrenal insufficiency and need corticosteroid support.
Emotional and Physical Impact
Women with an undiagnosed adrenal tumor often suffer deeply. They’re told their symptoms are due to PCOS, stress, or even imagined. They may feel dismissed, unheard, or lost in a medical system that overlooks rare conditions. The anxiety of not knowing what’s wrong—and the fear of infertility or serious disease—can be overwhelming.
That’s why an accurate diagnosis is powerful. It restores not just physical health, but emotional stability and confidence. Women regain trust in their bodies and in medicine.
How Often Does This Happen?
Adrenal tumors are not common, but they’re not rare either. They occur in up to 5% of the population, and many are found incidentally during imaging for unrelated issues. However, functioning adrenal tumors — those that secrete hormones — are less frequent but far more dangerous if missed.
Among women misdiagnosed with PCOS, a small percentage are later found to have an adrenal tumor. These cases are a critical reminder that every woman’s body is different and deserves a personalized approach.
The Role of Specialists
Not every OB-GYN is trained to recognize the signs of an adrenal tumor. That’s why referral to endocrinologists or reproductive endocrinologists is essential when symptoms don’t respond to PCOS treatment or lab values appear suspicious.
Specialists can interpret complex hormonal results, order appropriate imaging, and manage long-term care — from diagnosis to surgery and hormone regulation.
Ana’s Outcome: A Message of Hope
After her diagnosis, Ana underwent successful laparoscopic removal of her adrenal tumor. Her DHEA-S levels returned to normal within a month. Her periods resumed, her skin improved dramatically, and her confidence returned.
Ana’s story is one of thousands — but it underscores the importance of listening to your body, asking for thorough testing, and never accepting a label that doesn’t fit.
Conclusion: Not All That Looks Like PCOS Is PCOS
If your symptoms feel “too much” or came on too quickly, trust your instinct. An adrenal tumor may be silently altering your body from within. But with awareness, proper evaluation, and timely treatment, recovery is not only possible — it’s likely.
Don’t wait for answers. Advocate for yourself. Ask about your DHEA-S. Push for imaging. And know this: a misdiagnosis is not your fault — but finding the truth is your right.
Adrenal Tumor: Final Words
Ana’s body was whispering before it screamed.
Six months wasted under a wrong diagnosis.
Six months where a tumor grew silently.
Six months where hope could have faded.
But she chose to ask again.
She chose to listen harder.
And she found her answer — and her freedom.
Today, she isn’t just healed.
She’s a fighter, a reminder that your body knows — even when others don’t listen.
Never be afraid to seek another opinion.
Never be afraid to demand more for your health.
You are the expert of your own story.
References
- DOS ANJOS, Fernanda R. M.; GÓES, Fernando L.; NASCIMENTO, Rosângela P.; FREITAS, Danilo A. Adenoma gigante de glândula supra-renal: abordagem cirúrgica laparoscópica. Revista UNINGÁ Review, 2021, v. 36, n. 1, p. 01–06. https://doi.org/10.46311/2318-0579.36.eUJ4231
- SANTOS, Amanda R.; PEREIRA, Lucas M. Hidden Androgen-Secreting Tumor Masquerading as PCOS: A Case Report. Journal of Clinical Endocrinology, 2020. https://doi.org/10.1210/jc.2020-00234
- AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE (ASRM). Evaluation and Treatment of Androgen Excess Disorders. Fertility and Sterility Practice Committee Report, 2023. ASRM 2023 PDF
- NIH – NATIONAL INSTITUTES OF HEALTH. Endocrine Disorders and Their Impact on Women’s Health. NIH Reports, 2022. NIH 2022 Report